
It is widely known that placental mammals originated in the Mesozoic as small, unspecialized forms that can best be compared to the insectivores of the modern mammal fauna. Such shrew-like mammals are often imagined as the sole survivors of the events that wiped away the dinosaurs, and they are often portrayed as the kind of mammal typical of the following epoch, the Paleocene. While the mammal fauna of the Paleocene was much more varied than this simplified picture implies, insectivore-like mammals in fact made up a significant part of the earliest faunas of the Cenozoic. Before we have a closer look at those animals, some concepts about their classification need to be clarified first.
Traditionally, insectivores have been classified as a single
order of placental mammals, the Insectivora, which includes
familiar animals such as shrews, moles and hedgehogs as well as
exotic forms like the golden moles of Africa and the tenrecs of
Madagascar. As the name implies, these animals feed typically on
small invertebrates such as insects or worms, but in fact most
insectivores eat almost anything organic that can be stuffed into
their mouths and chewed (5). Although many insectivores have
evolved specializations of their own, features such as the very
small brain show that they are among the most primitive placental
mammals. Early researchers therefore assumed that insectivores
occupied a central position within the Placentalia and that they
represented the stock from which all other groups of placentals
evolved. As an increasing number of primitive fossil forms were
discovered that showed no obvious relationship to any of the more
advanced orders, those were included in the Insectivora by
default. The order Insectivora thus became a kind of wastepaper
basket for problematic, generally primitive placental mammals (6,
7).
This traditional concept of the Insectivora, which sees the group as the base of the radiation of placental mammals, has today been abandoned. Instead, it has been proposed that the living insectivores and their close fossil relatives form a separate branch on the evolutionary tree of the placental mammals, just like the rodents or the primates, and should not be mixed up with unrelated archaic mammals. To avoid confusion with the old concept of the Insectivora, a different name, Lipotyphla, is often used for the order containing the "true" insectivores, while the archaic forms are sometimes referred to as Proteutheria (6, 7). To make matters still more complicated, recent molecular studies have shown that certain insectivores from Africa and Madagascar, the golden moles and the tenrecs, are not closely related to the more "northern" insectivores. Instead, they are part of a typically African radiation of mammals, the Afrotheria, which also includes elephants, sea cows, hyraxes, aardvarks and elephant shrews. Golden moles and tenrecs have therefore been removed from the Lipotyphla and assigned to a new order, the Afrosoricida ("African shrews") (8, 9). For convenience, the term "insectivore" will be used here for insectivore-like placental mammals, no matter whether they are related to the Lipotyphla or Afrosoricida.
Figure 1: Reconstruction of Cimolestes, a primitive insectivore-like mammal mainly known from the Late Cretaceous to Early Paleocene of North America. Since little is known of this animal beyond its teeth, the reconstruction is largely hypothetical. From (61).
Since skeletal remains of insectivores are rather delicate, usually only their teeth and jaws are preserved as fossils, and even those are not easy to collect due to their tiny size. Insectivores tend therefore to be under-represented in collections of Early Tertiary mammals. This has improved since the 1960s by applying screen-washing techniques on fossil-bearing sediments (10). We now know that insectivores made up a significant component of the Paleocene faunas of North America, Europe and at least northern Africa (11, 12, 13, 14, 15). In a Middle Paleocene fauna from Montana containing 55 species of mammals, for instance, the 18 species of insectivores account for a third of the total species number (11). Insectivores were present in the Paleocene of Asia as well, but they appear to have been less diverse there (16). This may partly reflect a still insufficient knowledge of the small mammal fauna in that area. However, primitive relatives of rodents and rabbits known as anagalidans were highly diverse in the Asian Paleocene, and they may have filled some of the roles occupied elsewhere by insectivores. In contrast to the northern continents, insectivores are nearly absent in the Paleocene of South America, where marsupials occupied - and still occupy today - the corresponding ecological niches (17, 18). No Paleocene faunas are known from Australia and Antarctica (still ice-free then), which were at that time connected to each other and to South America. Later faunas give no indications that placental insectivores inhabited these areas in the Early Tertiary (19). We may therefore speculate that the situation there was similar to South America.
Insectivore-like mammals have long been known to occur in the Late Cretaceous. Cimolestes, for instance, flourished in the Latest Cretaceous of North America, and this archaic genus even made it into the Early Paleocene (5, 20). Most species of Cimolestes were mouse- to rat-sized, but the Late Cretaceous Cimolestes magnus reached the size of a marmot, making it one of the largest Mesozoic mammals known (5, 21). Although the classification of such early forms is debated, they are often assigned to the family Palaeoryctidae. The core members of the group occur in the Early Tertiary and include Palaeoryctes from the Middle to Late Paleocene of North America (5, 20, 22). Most species of Palaeoryctes ranged from about 20g to 60g in body weight, which puts their size somewhere in the range between mouse and chipmunk (1). While the cheek teeth of more generalized forms such as Cimolestes are still close to the basic structure of the most primitive placental mammals, combining the functions of piercing, shearing and grinding, the molars of Palaeoryctes had extremely high and acute cusps. They appear to have had little function other than piercing, as it is required for penetrating the tough carapaces of insects. When Palaeoryctes was first described, only a skull of the animal was known, so that the name Palaeoryctes ("early digger") was mainly based on speculation. Yet this name turned out to be excellently suited to its owner when a humerus fragment was discovered later, which showed clear evidence that Palaeoryctes had well-developed capabilities for digging (22).

Figure 2: Reconstruction of the shrew- to chipmunk-sized Palaeoryctes (Middle to Late Paleocene of North America and Late Paleocene of Africa), a typical member of the Palaeoryctidae showing adaptations for digging. From (62).
Although most palaeoryctids are known from North America and Europe, some members of the family have recently turned up in unexpected places. Both Cimolestes and Palaeoryctes are known from the single Paleocene fauna discovered in Africa, the localities of the Ouarzazate Basin in Morocco. The Ouarzazate species of Palaeoryctes is truly diminutive and may have weighed only 2g, about the weight of the pygmy shrew Sorex etruscus, one of the smallest living mammals (1). The occurrence of palaeoryctids in Northern Africa is not too surprising since the distance from Europe is not great, although the animals must somehow have crossed the ancient Tethys sea, the precursor of today's Mediterranean that separated Africa from Europe and Asia at that time (13, 23). Completely unexpected, however, was the recent discovery of an animal close to Cimolestes in the Early Paleocene of Bolivia. South America has been an isolated continent for most of the Tertiary, and no insectivore fossils had ever turned up from that period of isolation. So it had been assumed that insectivores had never entered the continent before the Late Cenozoic when the landbridge to North America was established. Shrews migrated into South America at that time, and they are still the only insectivores inhabiting South America today, limited in range to the northernmost part of the continent. Apart from this exception, the insectivore niche is occupied by the highly diverse marsupials in South America. The discovery of the Bolivian palaeoryctid shows that there had been competition between placentals and marsupials for the insectivore-niche in South America, and that the marsupials won it in the Early Tertiary (17, 18).
Forms such as the palaeoryctids illustrate that there is little difference between an insectivore and a small carnivorous mammal. The dentition of Cimolestes, in particular, foreshadows cutting structures seen in carnivores. While the smaller species appear to have been typical insectivores, the marmot-sized Cimolestes magnus probably took larger prey and may have been a carnivore to some degree. In fact it is possible that the two major groups of carnivorous placentals, the now extinct order Creodonta and the true carnivores of the order Carnivora, both evolved from forms close to Cimolestes (5, 24). Possible relatives of the Palaeoryctidae, the species Hyracolestes ermineus ("ermine-like shrew robber") and Sarcodon pygmaeus ("pygmy flesh tooth"), were common in the Latest Paleocene of Mongolia and China and may have partly occupied the small predator niche there. Hyracolestes probably weighed about 40g, Sarcodon about 75g, which is in the range of the smallest living weasels and ermines (1). As a telltale sign of the ferocious habits of its owner, the cheek teeth of Hyracolestes show characteristic notches that serve in today's carnivores to hold flesh in place as it is sheared apart by the cutting ridges (25, 26, 27).
The palaeoryctids may also have evolved into a quite different direction: The teeth of some of their members closely resemble those of an archaic order of mammals, the Taeniodonta, which includes relatively large animals that specialized in digging and rooting (28). The palaeoryctid Alveugena from the Early Paleocene of Wyoming has even be proposed as the "missing link" between both groups. This form is the largest known palaeoryctid, reaching the size of a small to medium dog (21). However, the recent discovery of a Mesozoic taeniodont, Schowalteria from Late Cretaceous of Alberta, has again raised doubts about this hypothesis. Apart from the fact that Schowalteria is significantly older than the supposed "missing link" Alveugena, the teeth of the taeniodont are also more primitive in certain aspects, suggesting that many (and perhaps all) of the similarities between early taeniodonts and palaeoryctids are the result of parallel evolution (29).
Although some scientists consider the palaeoryctids to be members of the Lipotyphla (i. e. true insectivores), this remains disputed (7). The Late Cretaceous Paranyctoides has been proposed as the oldest known lipotyphlan, and nothing precludes a Mesozoic origin of the order, but the evidence is still ambiguous. Undisputed members of the order Lipotyphla are first known from the Paleocene, and they already include members of the two major divisions of living lipotyphlans: Hedgehogs and kin on the one hand, and shrews and their relatives, including moles, on the other hand (30). Members of both groups are among the smallest mammals preserved in Paleocene faunas.
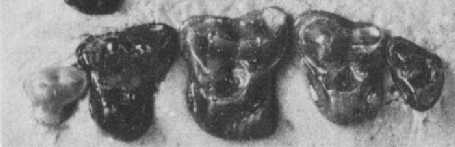
Figure 3: Upper cheek teeth of Adapisorex abundans, a mouse-sized member of the hedgehog lineage which was found in abundance in the Late Paleocene fissure fillings of Walbeck, Germany. Third premolar (left) to third molar (right) are shown, outer side is above. The relatively quadrate shape of their molars, the low cusps and the strongly reduced last molar are characteristic of the hedghehog family. Original image length 1cm. From (12).
Relatives of the hedgehogs, formally known as erinaceomorphs (from Erinaceus, the European hedgehog), appear first with Mackennatherium from the Middle Paleocene of North America and with the closely related Adunator from Germany, perhaps slightly younger in age (30, 31, 32). These were mouse-sized forms of about 10 to 20g body weight (1) that show less high-cusped cheek teeth than the palaeoryctids or many other insectivores. Their teeth closely resemble those of primitive hoofed mammals belonging to the family Hyopsodontidae, and the classification of several forms as either insectivores or archaic hoofed mammals is still disputed. Several Late Paleocene insectivores, such as Litolestes from Wyoming and Adapisorex from France and Germany, are sufficiently similar to hedgehogs to be classified in the same family, the Erinaceidae (31). This is in fact an exceptional case, since all other placental mammals of the Paleocene epoch, with the exception of armadillos, belong to families no longer existing today. Yet classification as members of the hedgehog family does not imply that those early insectivores had already evolved some hedgehog-like defense mechanism; not even all living members of the hedgehog family have spines. As exquisitely preserved fossils from the Grube Messel in Germany show, the erinaceomorph Macrocranion had evolved spines by the Middle Eocene (at least in one of the two species preserved at Messel, the second species having wooly hair) (33). The genus Macrocranion, which is also known by teeth from the Latest Paleocene of Wyoming (34), surprisingly shows elongated hindlimbs and a long tail. Apparently Macrocranion resorted to hopping on its hindlegs when on flight from a predator, just as some rodents do today - an unexpected mode of locomotion in a relative of the hedgehogs.
The oldest and most primitive shrew-relatives, or soricomorphs (from the shrew genus Sorex), are the Nyctitheriidae (30). The family appears first in North America with Leptacodon, shrew-sized animals of about 7 to 14g (1) that occur from the Early Paleocene to the Early Eocene, and the last known members of the family are Oligocene in age (20, 32). With the exception of a yet undescribed skeleton of Leptacodon from the Latest Paleocene of Wyoming, little is known of nyctitheriids beyond their jaws and teeth (34). Certain bones of the ankle have been described for late Eocene forms. Their structure suggests that the animals were tree-dwelling, but it also raises some doubts whether nyctitheriids were really members of the Lipotyphla (35). As for the dentition, nyctitheriids had particularly sharp cheek teeth and evolved incisors with several separate cusps, a feature that also occurs in today's shrews (30, 36). The upper molars of some advanced nyctitheriids show a W-shaped pattern called dilambdodonty. This pattern also characterizes the cheek teeth of shrews and moles, and it is possible that the Nyctitheriidae include the ancestors of both modern groups (6, 30). True shrews and moles do not occur before the Middle Eocene (32). Concerning moles, it is remarkable that unidentified animals with a comparable subterranean way of life must already have existed during the Paleocene epoch. An isolated humerus from the Middle Paleocene of Montana strongly resembles the highly specialized upper arm bone of moles, leaving no doubt that this early mammal had similar habits (37). The owner of the fossil humerus probably belongs to one of the groups of insectivore-like mammals discussed here, but identification will only be possible when such bones are found together with teeth.
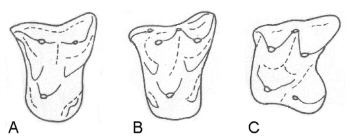
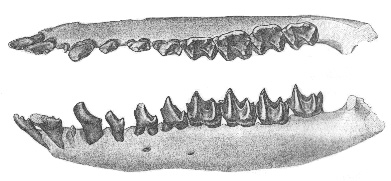
Figure 4 (left): Evolution of the dilambdodont (W-shaped) pattern in the upper molars of shrews and relatives. From the Early Tertiary nyctitheriids Leptacodon (A) and Pontifactor (B) to the living shrew genus Sylvisorex (C) the outer line of cusps evolved from a straight line into a W-shape. Front of molars is to the left, outer side above. Modified after (30).
Figure 5 (right): Nearly complete lower jaw of the nyctitheriid Ceutholestes dolosus from the Latest Paleocene of Wyoming, shown from above and outside. As in other members of the family, the incisors (partly damaged) end in several separate tips, and the cheek teeth have extremely sharp cusps. Total jaw length 17mm. From (36).
The Nyctitheriidae have also been proposed as relatives of another group of modern mammals, the bats of the order Chiroptera. Full-fledged bats appear all of a sudden in the fossil record in the Early Eocene, and they already show significant diversity by that time (38). This implies either an incredibly rapid evolution or, more probably, an origin of the order well in the Paleocene. Fossil skeletons of early bats should be easy to recognize by at least the beginning development of a flight apparatus, but unfortunately no such skeletons (which would only be preserved under exceptional circumstances) are known from the Paleocene. However, there are only small differences between the teeth of bats and those of certain Paleocene insectivores, including members of the Nyctitheriidae and an unnamed genus known from Germany and Rumania (38, 39, 40). Some of these animals may turn out to be ancient bats when they become better known, especially when skeletons are found. This would not be surprising from the point of view of molecular studies which place the orders Chiroptera and Lipotyphla close to each other on the family tree of mammals (7, 8).
Due to the scarcity of the African record of early Tertiary mammals, we have little clues about the early history of the endemic African insectivores of the order Afrosoricida. The hedgehog-like tenrecs, today restricted to Madagascar, and the burrowing golden moles are first known from the Miocene of Eastern Africa. Their upper teeth are characterized by a V-shaped pattern, a condition that is known as zalambdodonty (6). Two upper teeth of an unnamed animal from the Late Paleocene of Morocco are vaguely similar to the teeth of tenrecs and golden moles and may represent an early relative of these African insectivores. Given the fact that a gap of more than 33 million years separates these fragmentary fossils from the earliest tenrecs and golden moles, no conclusion is currently possible (15, 41). However, we must keep in mind that tenrecs and golden moles are part of the Afrotheria, the great African mammal radiation which also includes elephants, aardvarks, elephant shrews, hyraxes and sea cows. Elephants are first known from the Earliest Eocene of Morocco, and their group is certainly older (42). Splitting between the different groups of Afrotheria must therefore have started at least well within the Paleocene, making it is highly probable that the Afrosoricida are also an ancient group of mammals.
Besides animals related to the living groups of insectivores, Paleocene faunas contain a number of insectivore-like placental mammals that appear to belong to independent groups. As mentioned before, these groups have sometimes been included in the order Proteutheria. Despite their limited diversity it may be more appropriate to accommodate them in separate orders: the Leptictida, the Pantolesta and the Apatotheria (6).
 Members of the
Leptictida were originally regarded as archaic hedgehogs, a
notion that continues to live in popular reconstructions.
Although some scientists still tend to link these animals to the
true insectivores (7), they were in fact quite unlike hedgehogs
since they tended to increase the size of their hindlegs in
comparison to the front legs (43, 44). The order first appears in
the Late Cretaceous of western North America with Gypsonictops.
This genus is extremely primitive in sometimes retaining a fifth
premolar, while almost all other placental mammals have reduced
the number of premolars to four (6, 43). The core of the order
consists of the Leptictidae, a mainly North American family that
first occurs in the Early Paleocene with Prodiacodon and Palaeictops.
Prodiacodon ranges into the Early Eocene, while Palaeictops
occurs up to the Middle Eocene. The latter genus probably gave
rise to Leptictis, the last and best represented leptictid
for which about 200 skulls are known from Oligocene deposits (20,
43). Small species of Prodiacodon were mouse-sized with a
body weight of about 30g, whereas larger species of Prodiacodon
and Palaeictops attained over 200g and were comparable to
rats in size (1). While Prodiacodon still had teeth with
high and sharp cusps as they are typical of insectivorous
mammals, the cusps became lower and more rounded in Palaeictops,
suggesting a shift towards a more varied diet.
Members of the
Leptictida were originally regarded as archaic hedgehogs, a
notion that continues to live in popular reconstructions.
Although some scientists still tend to link these animals to the
true insectivores (7), they were in fact quite unlike hedgehogs
since they tended to increase the size of their hindlegs in
comparison to the front legs (43, 44). The order first appears in
the Late Cretaceous of western North America with Gypsonictops.
This genus is extremely primitive in sometimes retaining a fifth
premolar, while almost all other placental mammals have reduced
the number of premolars to four (6, 43). The core of the order
consists of the Leptictidae, a mainly North American family that
first occurs in the Early Paleocene with Prodiacodon and Palaeictops.
Prodiacodon ranges into the Early Eocene, while Palaeictops
occurs up to the Middle Eocene. The latter genus probably gave
rise to Leptictis, the last and best represented leptictid
for which about 200 skulls are known from Oligocene deposits (20,
43). Small species of Prodiacodon were mouse-sized with a
body weight of about 30g, whereas larger species of Prodiacodon
and Palaeictops attained over 200g and were comparable to
rats in size (1). While Prodiacodon still had teeth with
high and sharp cusps as they are typical of insectivorous
mammals, the cusps became lower and more rounded in Palaeictops,
suggesting a shift towards a more varied diet.
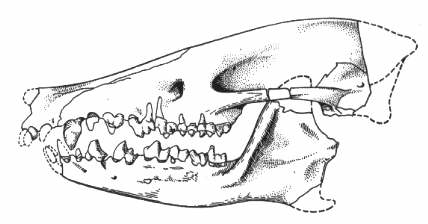
Figure 5: Skull of the Early Eocene leptictid Palaeictops bicuspis, showing rather low-cusped teeth. The genus Palaeictops ranges from the Early Paleocene to Middle Eocene of North America. Reconstructed skull length 65mm. From (63), after (64).
 Although a largely complete skeleton of
a leptictid is known from the Late Paleocene of Wyoming, this
material still remains to be described in detail (11, 44).
Fragmentary skeletons from the Early Eocene, representing both Prodiacodon
and Palaeictops, show that the forelimbs of these
leptictids were relatively short, while the hindlimbs were long
and slender. Two bones of the hind limb, the tibia and the
fibula, were fused for half of their length, a tendency that can
already be detected in Middle Paleocene bones of Prodiacodon,
although to much lesser degree. Fusion of these bones occurs in
mammals adapted for rapid locomotion since it reduces the mass of
the limb, while rotation and twisting of the foot are restricted.
As these fossils suggest, leptictids were living on the ground
and usually moved on all four legs but may have resorted to
hopping on their hindlegs at high speeds, much like the African
elephant-shrews of today. The forelimbs of Prodiacodon and
Palaeictops show adaptations for digging, suggesting that
these animals were burrowing (45, 46).
Although a largely complete skeleton of
a leptictid is known from the Late Paleocene of Wyoming, this
material still remains to be described in detail (11, 44).
Fragmentary skeletons from the Early Eocene, representing both Prodiacodon
and Palaeictops, show that the forelimbs of these
leptictids were relatively short, while the hindlimbs were long
and slender. Two bones of the hind limb, the tibia and the
fibula, were fused for half of their length, a tendency that can
already be detected in Middle Paleocene bones of Prodiacodon,
although to much lesser degree. Fusion of these bones occurs in
mammals adapted for rapid locomotion since it reduces the mass of
the limb, while rotation and twisting of the foot are restricted.
As these fossils suggest, leptictids were living on the ground
and usually moved on all four legs but may have resorted to
hopping on their hindlegs at high speeds, much like the African
elephant-shrews of today. The forelimbs of Prodiacodon and
Palaeictops show adaptations for digging, suggesting that
these animals were burrowing (45, 46).
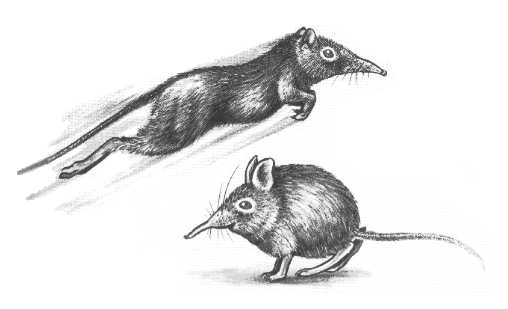
Figure 6: The living elephant-shrews (here the genus Elephantulus) may serve as a model for the locomotion of leptictids. Althoug elephant-shrews normally move on all four legs, they are able to leap on their long hindlegs if threatened by a predator. From (65).
Middle Eocene fossils document that the order Leptictida also included different adaptive types, although lengthening of the hindlimbs seems to be a common characteristic. Leptictidium from the Middle Eocene of Messel was a relatively large animal, reaching up to 90 cm in total length. Its tail was extremely long, accounting for 60 percent of the length, much more than in leptictids, and the size difference between front- and hindlegs was much more pronounced. The unusually elongated snout probably carried a small trunk in life. However, the most significant differences from leptictids are in the hindlimbs, which show no signs of bone fusion but retain full mobility. It seems that Leptictidium was not only capable of hopping but also of rapid running on its hindlegs, a mode of locomotion that has no close counterpart in the modern world. Fossilized stomach contents suggest that Leptictidium used this ability to pursue prey such as large insects and small reptiles and mammals. As for its ancestors, the known leptictids can be excluded since they are already specialized into a different direction (44). We must therefore assume long period of independent evolution reaching back into the Paleocene.
While the Leptictida dashed across the floor of the early Tertiary forests, members of the extinct order Pantolesta apparently took to the water. The core of the order consists of the Pantolestidae, a family which includes a sequence of forms with increasingly otter-like adaptations, starting with Middle Paleocene Bessoecetor and leading over Late Paleocene to Early Eocene Palaeosinopa to advanced forms such as the Middle Eocene Pantolestes and Buxolestes (12, 47, 48, 49). Bessoecetor and Palaeosinopa both appear first in North America, and Palaeosinopa spread into Europe at the beginning of the Eocene, a time of major faunal exchange between the northern continents. However, early pantolestids of the genus Pagonomus were already present in Europe by Late Paleocene time, which complicates speculations about the origin of the group. Pantolestids also reached Asia in the Eocene, where the last of the group endured until the Early Oligocene (12, 32).
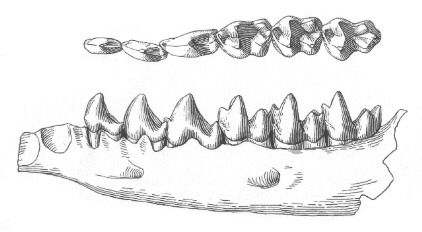
Figure 7: Lower dentition of Bessoecetor septentrionalis from the Late Paleocene of Montana, U. S. A, shown from above and outside. The genus Bessoecetor (also known as Propalaeosinopa) is the earliest known member of the semiaquatic Pantolestidae. Length of jaw about 17 mm. From (66).
Pantolestids were relative large for insectivore standards. Late Paleocene species of Palaeosinopa, for instance, may have reached body weights of 1400g, which is comparable to today's musk-rats (1). Some degree of aquatic adaptation has been suspected since 1909, when the skull and skeleton of Pantolestes were analyzed by the paleontologist William D. Matthew (50). The skull shows already a number of revealing traits, most of which can be traced back to the oldest known pantolestid skull, a Late Paleocene specimen of Palaeosinopa (48, 49). The Palaeosinopa skull has a long, low and flat-topped profile, a streamlined shape as it occurs today in otters and seals. The canines are moderately large, and the cheek teeth are massive with relatively low cusps. The skull provided ample space to accommodate a powerful jaw closing musculature. Together with the build of the teeth this has been interpreted as a beginning adaptation for crushing hard food such as clams or snails. As in other pantolestids, a certain opening (or foramen) of the skull is well developed below the orbit. Since this opening contains the blood-vessels and nerves for the snout, the animal probably had a well-developed tactile snout, which would help for underwater feeding. Later pantolestids show in addition a prominent crest at the back of the skull, as well as strong bone processes of the second neck vertebra, which indicates strong neck musculature. This is required for a swimmer that constantly needs to hold its neck up (33, 51, 52).
 Since no Paleocene skeleton of a
pantolestid is known up to now, we must rely on the evidence
supplied by Eocene representatives. Excellently preserved
skeletons from the Middle Eocene of Messel are available for the
pantolestid Buxolestes, which is represented by two
different species. The larger species had a head and body length
of 46 cm, with an additional 35 cm for the tail, which is still
significantly smaller than most living species of otters.
Forelimbs and hindlimbs are powerfully built but show no
unambiguous specializations for swimming. They end in strong
claws, which may indicate the capability of building burrows. It
is in the tail that we find clear evidence for swimming habits:
The first tail vertebrae show particularly widened processes on
their sides, which must have served as attachment for powerful
musculature to move the tail, apparently to generate propulsion
in water. As a final confirmation of the semiaquatic habits of Buxolestes,
several individuals have preserved remains of fish in their
stomach contents. Remains of other kinds of food are present as
well, but apparently the Messel population of Buxolestes
did not show the predilection for clams or snails that had been
postulated from the dentition. The resulting picture is that of
an animal which spent at least much time in the water catching
fish, but which was not too specialized and must have been agile
on land as well (33, 51, 52).
Since no Paleocene skeleton of a
pantolestid is known up to now, we must rely on the evidence
supplied by Eocene representatives. Excellently preserved
skeletons from the Middle Eocene of Messel are available for the
pantolestid Buxolestes, which is represented by two
different species. The larger species had a head and body length
of 46 cm, with an additional 35 cm for the tail, which is still
significantly smaller than most living species of otters.
Forelimbs and hindlimbs are powerfully built but show no
unambiguous specializations for swimming. They end in strong
claws, which may indicate the capability of building burrows. It
is in the tail that we find clear evidence for swimming habits:
The first tail vertebrae show particularly widened processes on
their sides, which must have served as attachment for powerful
musculature to move the tail, apparently to generate propulsion
in water. As a final confirmation of the semiaquatic habits of Buxolestes,
several individuals have preserved remains of fish in their
stomach contents. Remains of other kinds of food are present as
well, but apparently the Messel population of Buxolestes
did not show the predilection for clams or snails that had been
postulated from the dentition. The resulting picture is that of
an animal which spent at least much time in the water catching
fish, but which was not too specialized and must have been agile
on land as well (33, 51, 52).
The evidence from Messel has recently been supplemented by a remarkable skeleton of Palaeosinopa from the Early Eocene of Wyoming. The new specimen of this ancient pantolestid genus comes from the Fossil Butte Member, a rock formation which is world-famous for its abundant fish fossils but has just recently started to yield rare mammal fossils. With a head and body length of 57cm, this animal is closer in size to today's otters than Buxolestes. Apart from the somewhat more gracile build and the longer, more slender tail, the skeletons of Palaeosinopa and Buxolestes are very similar in general proportions. Both genera share the same basic adaptations to a semiaquatic way of life. In addition, the feet of Palaeosinopa are very large and must have been well suited for generating propulsion during swimming. Last but not least, fossilized stomach contents of Palaeosinopa show that it resembled Buxolestes in its propensity for fish (52).
Figure 8: Skeleton of the otter-like pantolestid Palaeosinopa, a genus which first appears in the Late Paleocene, from the Early Eocene Fossil Butte Member of Wyoming. Several adaptations of the skeleton, including the large feet, show that this animal was a proficient swimmer. Photo G. Oleschinski, provided by W. von Koenigswald.
Other members of the Pantolesta are the pentacodontids, a small North American group sometimes regarded as a subfamily of the Pantolestidae. Typical members are Pentacodon and Aphronorus from the Middle to Late Paleocene of the Western Interior. The latter genus is known from rather complete skull material (20, 32, 53). Like other members of the order, some pentacodontids got unusually large for insectivore-like mammals. The largest species of Pentacodon may have weighed about 3400 g, comparable to a small marmot, and there is evidence for an even larger form (1, 54). Pentacodontids are characterized by enlarged, puncturing and crushing fourth premolars, and their teeth are often heavily worn, suggesting that they took in large amounts of grit during feeding. Like in the case of the pantolestids, it has been proposed that they specialized on hard-shelled food such as snails, but they were probably at home on dry ground (53).
 An unusual specialization has recently been
documented in Bisonalveus browni, a small pentacodontid of
about 65g (somewhat above mouse size (1)) that was first
described from the Late Paleocene of Wyoming. While the first
finds of Bisonalveus were limited to jaw fragments, an
incomplete skull was discovered later in Alberta, Canada. It
contained the first upper canines known for Bisonalveus,
which are long dagger-like teeth with a peculiar groove running
down in front. Similar grooves exist in the poison fangs of
modern snakes, in which they serve for conducting venom produced
in glands in the upper jaw. Apparently Bisonalveus had a
similar venom delivery system which the tiny animal used to
immobilize its prey, mainly insects and other invertebrates. Only
few kinds of living mammals, including a number of shrew species
and Solenodon, an insectivore only known from the
Caribbean islands, are known to have a poisonous bite, and no
comparable case was previously known for any extinct kind of
mammal. It is therefore astonishing that isolated canines of
another, larger mammal from the Late Paleocene of Alberta, still
unidentified as to affinities, also show a groove that must have
served for conducting venom. Unlike the poison fangs of Bisonalveus
these teeth are from the lower jaw, and they are very differently
built, which shows that the capability to use venom was evolved
independently in these animals. This suggests that venomous
mammals may have been more diverse in the early Cenozoic than
they are today (55).
An unusual specialization has recently been
documented in Bisonalveus browni, a small pentacodontid of
about 65g (somewhat above mouse size (1)) that was first
described from the Late Paleocene of Wyoming. While the first
finds of Bisonalveus were limited to jaw fragments, an
incomplete skull was discovered later in Alberta, Canada. It
contained the first upper canines known for Bisonalveus,
which are long dagger-like teeth with a peculiar groove running
down in front. Similar grooves exist in the poison fangs of
modern snakes, in which they serve for conducting venom produced
in glands in the upper jaw. Apparently Bisonalveus had a
similar venom delivery system which the tiny animal used to
immobilize its prey, mainly insects and other invertebrates. Only
few kinds of living mammals, including a number of shrew species
and Solenodon, an insectivore only known from the
Caribbean islands, are known to have a poisonous bite, and no
comparable case was previously known for any extinct kind of
mammal. It is therefore astonishing that isolated canines of
another, larger mammal from the Late Paleocene of Alberta, still
unidentified as to affinities, also show a groove that must have
served for conducting venom. Unlike the poison fangs of Bisonalveus
these teeth are from the lower jaw, and they are very differently
built, which shows that the capability to use venom was evolved
independently in these animals. This suggests that venomous
mammals may have been more diverse in the early Cenozoic than
they are today (55).
Figure 9: Upper Canine (right) and third upper incisor (left) of the Late Paleocene pentacodontid Bisonalveus browni in front view. A deep groove running down the length of the canine must have functioned as a gutter, conducting poison to the tip of the tooth in order to kill or immobilize the animal's prey. Scale bar 1mm. Photo provided by C. S. Scott.
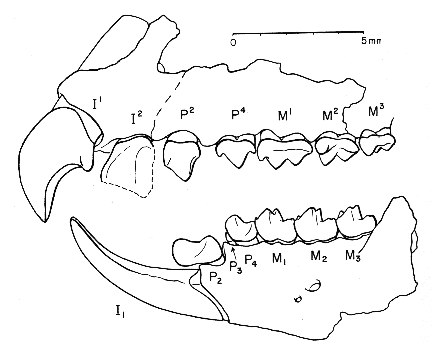
The last group of insectivore-like mammals discussed here, the order Apatotheria, has been a mystery to scientists for many years. The single family of the order, the Apatemyidae, is characterized by a very specialized dentition, which includes a pair of strongly enlarged incisors. Similarly enlarged front teeth occur in a number of Early Tertiary primate-like mammals such as the Plesiadapidae, which has led to frequent confusion of the groups in their early history of study. It is therefore not surprising that the apatemyids have often been regarded as primates in the past, a view which is no longer maintained today (56). Although relationships to diverse orders of mammals have been proposed, it seems at present most practical to classify the apatemyids in their own order Apatotheria (6).
 As far as we know, apatemyids only
occurred in the Early Tertiary of North America and Europe (32).
The first unambiguous apatemyids appear in the Middle Paleocene
of the Rocky Mountain region with the extremely primitive Unuchinia
and the somewhat more advanced Jepsenella. The latter
was followed by Labidolemur in the Late Paleocene (56,
57). While species of Unuchinia weighed up to 80g, Jepsenella
and Labidolemur were only mouse-sized with body weights
between 15 and 40g (1). Labidolemur had essentially
evolved the dental architecture that characterizes the family.
There are two upper incisors on each side of the jaw, the first
of them greatly enlarged and hook-shaped. The single enlarged
lower incisor runs forward from the jaw and then curves upward to
a sharply pointed tip, forming a spoon-shape tool with the tooth
in the other jaw side. In total, the arrangement of the front
teeth looks vaguely rodent-like. All other incisors and the
canines have been lost, as have been the first premolars. Among
the cheek teeth, the second lower premolar is strikingly
modified: Its wedge-shaped crown is projecting far forward, most
of it overhanging the base of the lower incisor (56, 57).
As far as we know, apatemyids only
occurred in the Early Tertiary of North America and Europe (32).
The first unambiguous apatemyids appear in the Middle Paleocene
of the Rocky Mountain region with the extremely primitive Unuchinia
and the somewhat more advanced Jepsenella. The latter
was followed by Labidolemur in the Late Paleocene (56,
57). While species of Unuchinia weighed up to 80g, Jepsenella
and Labidolemur were only mouse-sized with body weights
between 15 and 40g (1). Labidolemur had essentially
evolved the dental architecture that characterizes the family.
There are two upper incisors on each side of the jaw, the first
of them greatly enlarged and hook-shaped. The single enlarged
lower incisor runs forward from the jaw and then curves upward to
a sharply pointed tip, forming a spoon-shape tool with the tooth
in the other jaw side. In total, the arrangement of the front
teeth looks vaguely rodent-like. All other incisors and the
canines have been lost, as have been the first premolars. Among
the cheek teeth, the second lower premolar is strikingly
modified: Its wedge-shaped crown is projecting far forward, most
of it overhanging the base of the lower incisor (56, 57).
Figure 9: Dentition of Labidolemur kayi, an apatemyid from the Late Paleocene to Early Eocene of North America, showing the greatly enlarged first incisors and the blade-like second lower premolar. From (57).
For what could such a highly specialized dentition be used? Since a long time, scientists have noted that the front teeth of the apatemyids are similar to those of two living mammals: The aye-aye of the genus Daubentonia, a bizarre lemur living in Madagascar, and the striped possum Dactylopsila, a marsupial inhabiting New Guinea. Both animals use their enlarged incisors to tear off bark in order to get access to wood-boring insects such as beetle larvae. In fact, both animals are the ecological equivalents of woodpeckers, a group of birds that is absent in Madagascar and New Guinea (but present nearly everywhere else). Given the close similarity of the dentition, it appeared possible that apatemyids took over a similar ecological role in the Early Tertiary (58).
Testing this hypothesis became possible with the discovery of complete skeletons of apatemyids. As so often, the breakthrough happened in Messel, which yielded the first skeletons of the Middle Eocene apatemyid Heterohyus in the 1970s and 1980s (33, 51, 58). They belong to delicate animals about the size of a dormouse, with a head and body length of about 13cm and a tail of 16cm. Just as in the case of the pantolestids, another well-preserved apatemyid skeleton has recently been found in the Early Eocene sediments of the Fossil Butte Member, Wyoming (52, 59). It belongs to Apatemys, a predominantly Eocene genus (although some researchers include the Late Paleocene species of Labidolemur in this genus as well). Regarding head and body length this animal was only little larger than the Messel specimens, but its tail was much longer. Finally, two nearly complete skeletons of Labidolemur have recently been discovered in Late Paleocene and Early Eocene freshwater limestones of the Clarks Fork Basin, also in Wyoming (34, 60).

Figure 10: Skeleton of the apatemyid Apatemys chardini, preserved together with the fish Knightia eocaenica in the Early Eocene lake sediments of the Fossil Butte Member, Wyoming. The skull is surprisingly masssive for such a delicate skeleton, and the tail is extremely long. Photo J. Weinstein, provided by W. von Koenigswald.
 The different skeletons confirm, first
of all, that the apatemyids were specialized for life in the
trees. This can be inferred from the well-developed, flattened
claws, which anchored the animal firmly, and the long tail (59%
of the total length in Apatemys), which must have helped
in balancing. At least in Heterohyus, modified ankle
joints probably enabled the animal to descend trees head-first,
like today's squirrels. The most unusual feature of the apatemyid
skeletons, however, are the lengthened second and third fingers
of the hand. This adaptation is most extreme in Heterohyus.
Labidolemur shows roughly the same degree of finger
elongation as Apatemys, despite its older age. As a matter
of fact, the two living mammals with which the apatemyids had
previously been compared, the aye-aye and the striped possum,
both have lengthened particular fingers of the hand, which they
use to extract larvae from their burrows or from narrow crevices.
This last piece of evidence finally demonstrates that apatemyids
must have pursued a closely similar life style, which was
apparently already established by Late Paleocene time. The
astonishing fact that three unrelated kinds of mammals developed
the same distinctive complex of enlarged incisors and lengthened
fingers testifies to the power of convergent evolution. As often
in such cases, different groups of animals choose slightly
different solutions in detail. While the apatemyids have
lengthened the second and third finger, the striped possum has
only emphasized the third one. The aye-aye, on the other hand,
uses its extremely thin lengthened third finger for extracting
insects. When not in use, this finger is supported by the much
thicker fourth finger of equal length. Interestingly, the aye-aye
also uses its lengthened finger to tap the tree surface in order
to locate larvae under the bark, much like woodpeckers use their
bill, a technique known as percussive foraging. Whether
apatemyids ever evolved such techniques remains open to
speculation (33, 51, 52, 58, 59, 60).
The different skeletons confirm, first
of all, that the apatemyids were specialized for life in the
trees. This can be inferred from the well-developed, flattened
claws, which anchored the animal firmly, and the long tail (59%
of the total length in Apatemys), which must have helped
in balancing. At least in Heterohyus, modified ankle
joints probably enabled the animal to descend trees head-first,
like today's squirrels. The most unusual feature of the apatemyid
skeletons, however, are the lengthened second and third fingers
of the hand. This adaptation is most extreme in Heterohyus.
Labidolemur shows roughly the same degree of finger
elongation as Apatemys, despite its older age. As a matter
of fact, the two living mammals with which the apatemyids had
previously been compared, the aye-aye and the striped possum,
both have lengthened particular fingers of the hand, which they
use to extract larvae from their burrows or from narrow crevices.
This last piece of evidence finally demonstrates that apatemyids
must have pursued a closely similar life style, which was
apparently already established by Late Paleocene time. The
astonishing fact that three unrelated kinds of mammals developed
the same distinctive complex of enlarged incisors and lengthened
fingers testifies to the power of convergent evolution. As often
in such cases, different groups of animals choose slightly
different solutions in detail. While the apatemyids have
lengthened the second and third finger, the striped possum has
only emphasized the third one. The aye-aye, on the other hand,
uses its extremely thin lengthened third finger for extracting
insects. When not in use, this finger is supported by the much
thicker fourth finger of equal length. Interestingly, the aye-aye
also uses its lengthened finger to tap the tree surface in order
to locate larvae under the bark, much like woodpeckers use their
bill, a technique known as percussive foraging. Whether
apatemyids ever evolved such techniques remains open to
speculation (33, 51, 52, 58, 59, 60).
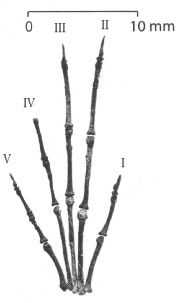
Figure 11: Hand of Labidolemur kayi, assembled from a nearly complete skeleton from the Latest Paleocene of Wyoming. Digits I (corresponding to the thumb) to V are marked, with the claw of digit IV not preserved. As in other apatemyids, digits II and III are lengthened to function as tools for extracting insects from crevices. Modified after (34).
The high degree of specialization of the apatemyids also explains why they are generally rare members of early Tertiary faunas (57). Of course, some variation of the mode of life must be expected within the group, especially since later forms reached larger size. The jaw of a Late Eocene species of Heterohyus may have been five times larger than those of the Messel skeletons. Like the aye-aye, which has a body mass of 2-3kg, large apatemyids probably used other resources such as seeds in addition to insects. Nevertheless, woodpeckers would have been important competitors of these unusual mammals. The first members of the woodpecker group are known from the Messel deposits, but these birds did not yet show the specializations of today's woodpeckers. A probable woodpecker's hole has been found in fossil wood from the Eocene of Arizona. Therefore it remains a plausible scenario that the spread of woodpeckers caused the extinction of the apatemyids in the Early Oligocene (33, 51, 52, 59).
Insectivore-like mammals are still among the least understood members of early Tertiary faunas. However, it often turns out that the common idea of these animals as generalized, rather featureless shrew- or rat-like forms is wrong when more complete remains of these animals are found. Like today's insectivores, these early Tertiary forms had some remarkable specializations and often pursued their own distinctive mode of life.
The extinction of dinosaurs clearly opened new opportunities for placental mammals, yet it is a little astonishing that there was only limited diversification of insectivore-like forms before the Paleocene. Predation pressure by dinosaurs is often cited as an explanation, but this is not fully convincing in the case of small mammals. Insectivores thrive in a world full of predators today, and there is no reason to suspect that dinosaurs were a greater threat to early insectivore-like mammals than carnivores or birds of prey are to small mammals today. We must therefore assume that some of the ecological niches later filled by insectivore-like placental mammals were occupied by other animals in the Late Cretaceous communities. Small carnivorous dinosaurs may have competed with some types of insectivore-like mammals (6) and may have been a limiting factor, as may have been insect-eating pterosaurs, especially in the trees. But even more important competitors were probably the marsupials, which were abundant and diverse in the Latest Cretaceous of North America but nearly went extinct there at the Cretaceous/Tertiary boundary. Of course, the history of mammals took a different turn in South America and Australia, where marsupials reign supreme in the insectivore niche to this day.
First upload 12.08.05. Special thanks to W. von Koenigswald for permission to use photos of the Palaeosinopa and Apatemys skeletons, and to C. S. Scott for providing the photo of the Bessoecetor canine.
References and notes:
(1) Body mass estimates are based on the size of the first lower molar, using published measurements of a diverse range of species and the approximation for insectivores published in (2). In case of the unusually large Pantolesta, an approximation for all mammals was used which is published in (3). Body weights of recent mammals were taken from different sources, including (4).
(2) Bloch, J. I., Rose, K. D. & Gingerich, P. D. 1998: New species of Batodonoides (Lipotyphla, Geolabididae) from the early Eocene of Wyoming: smallest known mammal? Journal of Mammalogy 79, 804-827
(3) Legendre, S. 1986: Analysis of mammalian communities from the late Eocene and Oligocene of southern France. Palaeovertebrata 16, 191-212
(4) Eisenberg, J. F. (Ed.) 1981: The Mammalian Radiations. The Athlone Press, London
(5) Kielan-Jaworowska, Z., Bown, T. M. & Lillegraven, J. A. 1979: Eutheria. In: Lillegraven, J.A., Kielan-Jaworowska, Z. & Clemens, W. (eds.): Mesozoic Mammals. The first two-thirds of mammalian history. Universityof California Press
(6) Carroll, R. L. 1988: Vertebrate paleontology and evolution. Freeman and Company.
(7) MacPhee, R. D. E. & Novacek, M. J. 1993: Definition and Relationships of Lipotyphla. In: Szalay, F. S., Novacek, M. J. & McKenna, M. C. (Eds.): Mammal Phylogeny. Placentals. Springer Verlag, 13 - 31
(8) Murphy, W. J. et al. 2001: Molecular phylogenetics and the origins of placental mammals. Nature 409, 614-618
(9) Madsen, O. et al. 2001: Parallel adaptive radiations in two major clades of placental mammals. Nature 409, 610-614
(10) Krause, D. W. 1984: Mammalian Evolution in the Paleocene: Beginning of an Era. In: Gingerich, P. D. & Badgley, C. E. (eds.): Mammals. Notes for a short course. University of Tennessee, Department of Geological Sciences, Studies in Geology 8, 87-109
(11) Rose, K. D. 1981: The Clarkforkian land-mammal age and mammalian faunal composition across the Paleocene-Eocene boundary. University of Michigan, Papers on Paleontology, v. 26, p. 1-197
(12) Russell, D. E. 1964: Les mammifères paléocènes d'Europe. Mémoires du Muséum National d'Histoire Naturelle, nouvelle série, série C, vol. 13, 1-324
(13) Gheerbrant, E. 1992: Les mammifères paléocènes du bassin d'Ouarzazate (Maroc). I. Introduction générale et Palaeoryctidae. Palaeontographica A Bd. 224, 67-132
(14) Gheerbrant, E. 1994: Les mammifères paléocènes du bassin d'Ouarzazate (Maroc). II. Todralestidae (Proteutheria, Eutheria). Palaeontographica A Bd. 231, 133-188
(15) Gheerbrant, E. 1995: Les mammifères paléocènes du bassin d'Ouarzazate (Maroc). III. Adapisoriculidae et autres mammifères (Carnivora, ?Creodonta, Condylarthra, ?Ungulata et incertae sedis). Palaeontographica A Bd. 237, 39-132
(16) Ting, S. 1998: Paleocene and Early Eocene Land Mammal Ages of Asia. In: Beard & Dawson (eds.): Dawn of the Age of Mammals in Asia. Bulletin of Carnegie Museum of Natural History 34, 124-147
(17) Marshall, L. G. & de Muizon, C. 1988: The Dawn of the Age of Mammals in South America. National Geographic Research 4(1), 23-55
(18) Van Valen, L. M. 1988: Faunas of a southern world. Nature 333, 113
(19) Vizcaíno, S. F., Reguero, M. A., Goin, F. J., Tambussi, C. P. & Noriega, J. I. 1998: Community structure of Eocene terrestrial vertebrates from Antarctic Peninsula. In: Paleógeno de América del Sur y de la Península Antártica. Asociación Paleontológica Argentina, publicación especial 5, 177-183
(20) Lofgren, D. L., Lillegraven, J. A., Clemens, W. A., Gingerich, P. D. & Williamson, T. E. 2004: Paleocene Biochronology: The Puercan Through Clarkforkian Land Mammal Ages. In: Woodburne, M. O. (ed.): Late Cretaceous and Cenozoic Mammals of North America. Biostratigraphy and geochronology. Columbia Univ. Press
(21) Eberle, J. J. 1999: Bridging the transition between didelphodonts and taeniodonts. Journal of Paleontology 73(5), 936-944
(22) Van Valen, L. 1966: Deltatheridia, a new order of mammals. Bulletin of the American Museum of Natural History 132, 1-126
(23) Gheerbrant, E. 1990: On the early biogeographical history of the African placentals. Historical Biology 4, 107-116
(24) Hunt, R. M. & Tedford, R. H. 1993: Phylogenetic Relationships Within the Aeluroid Carnivora and Implications of Their Temporal and Geographic Distribution. In: Szalay, F. S., Novacek, M. J. & McKenna, M. C. (Eds.): Mammal Phylogeny. Placentals. Springer Verlag, 53-73
(25) Matthew, W. D. & Granger, W. 1925: Fauna and correlation of the Gashato fauna of Mongolia. American Museum Novitates 189, 1-12
(26) Szalay, F. S. & McKenna, M. C. 1971: Beginning of the age of mammals in Asia: The Late Paleocene Gashato fauna, Mongolia. Bulletin of the American Museum of Natural History 144, 269-318
(27) Meng, J., Zhai, R. & Wyss, A. R. 1998: The Late Paleocene Bayan Ulan fauna of Inner Mongolia, China. In: Beard & Dawson (eds.): Dawn of the Age of Mammals in Asia. Bulletin of Carnegie Museum of Natural History 34, 148-185
(28) Schoch, R. M. 1986: Systematics, Functional Morphology and Macroevolution of the Extinct Mammalian Order Taeniodonta. Yale University Peabody Museum of Natural History Bulletin 42, 1-307
(29) Fox, R. C. & Naylor, B. G. 2003: A Late Cretaceous taeniodont (Eutheria, Mammalia) from Alberta, Canada. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 229(3), 393-348
(30) Butler, P. M. 1988: Phylogeny of the insectivores. In: Benton, M. J. (Ed.): The Phylogeny and Classification of the Tetrapods, Volume 2: Mammals, Clarendon Press, 117-141
(31) Novacek, M. J., Bown, T. M. & Schankler, D. 1985: On the Classification of the Early Tertiary Erinaceomorpha (Insectivora, Mammalia). American Museum Novitates 2813, 1-22
(32) McKenna, M. C. & Bell, S. K. 1997: Classification of mammals above the species level. Columbia University Press
(33) Koenigswald, W. et. al. 1998: Messel. Ein Pompeji der Paläontologie. Verlag Thorbecke, Sigmaringen.
(34) Bloch, J. I. & Boyer, D. 2001: Taphonomy of small mammals in freshwater limestones from the Paleocene of the Clarks Fork Basin. In: Gingerich, P. D. (ed.): Paleocene-Eocene Stratigraphy and Biotic Change in the Bighorn and Clarks Fork Basins, Wyoming. University of Michigan Papers on Paleontology 33, 185-198
(35) Hooker, J. J. 2001: Tarsals of the extinct insectivoran family Nyctitheriidae (Mammalia): Evidence for archontan relationships. Zoological Journal of the Linnean-Society 132(4), 501-529
(36) Rose, K. D. & Gingerich, P. D. 1987: A new insectivore from the Clarkforkian (Earliest Eocene) of Wyoming. Journal of Mammalogy 68(1), 17-27
(37) Simpson, G. G. 1937: The Fort Union of the Crazy Mountain Field, and its mammalian faunas. United States National Museum Bulletin 169, 1-287
(38) Hooker, J. J. 1996: A primitive emballonurid bat (Chiroptera, Mammalia) from the Earliest Eocene of England. Palaeovertebrata 25(2-4), 287-300
(39) Gheerbrant, E., Codrea, V., Hosu, A., Sen, S., Guernet, C., de Lapparent de Broin, F. & Riveline, J. 1999: Découverte de vertébrés dans les Calcaires de Rona (Thanétien ou Sparnacien), Transylvanie, Roumanie: les plus anciens mammifères cénozoiques d'Europe Orientale. Eclogae geologicae Helvetiae 92(3), 517-535
(40) Gingerich, P. D. 1987: Early Eocene bats (Mammalia, Chiroptera) and other vertebrates in freshwater limestones of the Willwood Formation, Clark's Fork Basin, Wyoming. Contributions from the Museum of Paleontology, University of Michigan 27, 275-320
(41) Gheerbrant, E., Sudre, J., Sen, S., Abrial, C., Marandat, B., Sigé, B. & Vianey-Liaud, M. (1998): Nouvelles données sur les mammifères du Thanétien et de l'Yprésien du Bassin d'Ouarzazate (Maroc) et leur contexte stratigraphique. Palaeovertebrata 27 (3-4), 155-202
(42) Gheerbrant, E., Sudre, J., Cappetta, H., Iarochène, M., Amaghzaz, M. & Bouya, B. 2002: A new large mammal from the Ypresian of Morocco: Evidence of surprising diversity of early proboscideans. Acta Palaeontologica Polonica 47(3), 493–506
(43) Novacek, M. J. 1977: A review of Paleocene and Eocene Leptictidae (Eutheria: Mammalia) from North America. PaleoBios 24, 1-42
(44) Storch, G. & Lister, A. M. 1985: Leptictidium nasutum, ein Pseudorhyncocyonide aus dem Eozän der "Grube Messel" bei Darmstadt (Mammalia, Proteutheria). Senckenbergiana lethaea 66, 1-37
(45) Rose, K. D. 1999: Postcranial skeleton of Eocene leptictidae (Mammalia), and its implications for behavior and relationships. Journal of Vertebrate Paleontology 19(2), 355-372
(46) Rose, K. D. 2001: Compendium of Wasatchian mammal postcrania from the Willwood Formation of the Bighorn Basin. In: Gingerich, P. D. (ed.): Paleocene-Eocene Stratigraphy and Biotic Change in the Bighorn and Clarks Fork Basins, Wyoming. University of Michigan Papers on Paleontology 33, 157-183
(47) Scott, C. S., Fox, R. C. & Youzwyshyn, G. P. 2002: New earliest Tiffanian (late Paleocene) mammals from Cochrane 2, southwestern Alberta, Canada. Acta Palaeontologica Polonica 47(4), 691-704.
(48) Dorr, J. A. 1977: Partial skull of Palaeosinopa simpsoni (Mammalia, Insectivora), Latest Paleocene Hoback Formation, Central Western Wyoming, with some general remarks on the family Pantolestidae. Contributions from the Museum of Paleontology, University of Michigan 24, 281-307
(49) Gingerich, P. D. 1980: A new species of Palaeosinopa (Insectivora: Pantolestidae) from the Late Paleocene of Western North America. Journal of Mammalogy 61(3), 449-454
(50) Matthew, W. D. 1909: Carnivora and Insectivora of the Bridger basin, middle Eocene. American Museum of Natural History Memoirs 9, 289-567
(51) Schaal, S. & Ziegler, W. (editors) 1988: Messel. Ein Schaufenster in die Geschichte der Erde und des Lebens. Verlag W. Kramer, Frankfurt am Main. (English edition: Messel. An Insight into the History of Life and of the Earth.)
(52) Von Koenigswald, W., Rose, K. Grande, L. & Martin, R. 2005: Die Lebensweise eozäner Säugetiere (Pantolestidae und Apatemyidae) aus Messel (Europa) im Vergleich zu neuen Skelettfunden aus dem Fossil Butte Member von Wyoming (Nordamerika). Geologisches Jahrbuch Hessen 132, 43-54
(53) Gingerich, P. D., Houde, P. & Krause, D. W. 1983: A new earliest Tiffanian (Late Paleocene) mammalian fauna from Bangtail Plateau, Western Crazy Mountain Basin, Montana. Journal of Paleontology 57, 957-970
(54) Williamson, T. E. 1996: The Beginning of the Age of Mammals in the San Juan Basin, New Mexico: Biostratigraphy and Evolution of Paleocene Mammals of the Nacimiento Formation. New Mexico Museum of Natural History and Science Bulletin 8, 1-141
(55) Fox, R. C. & Craig, S. S. 2005: First evidence of a venom delivery apparatus in extinct mammals. Nature 435, 1091-1093
(56) McKenna, M. C. 1963: Primitive Paleocene and Eocene Apatemyidae (Mammalia, Insectivora) and the Primate-Insectivore Boundary. American Museum Novitates 2160, 1-39
(57) Gingerich, P. D. & Rose, K. D. 1982: Dentition of Clarkforkian Labiolemur. Contributions from the Museum of Paleontology, University of Michigan 26, 49-55
(58) Koenigswald, W. v. & Schierning, H.-P. 1987: The ecological niche of an extinct group of mammals, the early Tertiary apatemyids. Nature 326, 595-597
(59) Koenigswald, W. v., Rose, K. D., Grande, L. & Martin, R. D. 2005: First Apatemyid Skeleton from the Lower Eocene Fossil Butte Member, Wyoming (USA), Compared to the European Apatemyid from Messel, Germany. Palaeontographica Abt. A 272, 149-169
(60) Bloch, J., Boyer, D., Silcox, M. & Houde, P. 2004: New skeletons of Paleocene-Eocene Labidolemur kayi (Mammalia, Apatemyidae): Ecomorphology and relationship of apatemyids to primates and other mammals (Abstract). Journal of Vertebrate Paleontology 24(3), 40A
(61) Savage, R. J. G. & Long, M. R. 1986: Mammal evolution. An illustrated guide. British Museum (Natural History)
(62) Cox, B. (ed.) 1988: Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. Macmillan London Limited.
(63) Piveteau, J. (ed.) 1961: Traité de Paléontologie. Tome VI: L'origine des mammifères et les aspects fondamentaux de leur évolution
(64) Matthew, W. D. & Granger, W. 1915: A revision of the Lower Eocene Wasatch and Wind River Faunas. Part V: Insectivora (continued), Glires, Edentata. Bulletin American Museum of Natural History 34, 429-483
(65) Grzimek, B. (ed.) 1988: Grzimeks Enzyklopädie Säugetiere. Band 2. Kindler Verlag
(66) Simpson, G. G. 1936: A new fauna from the Fort Union of Montana. American Museum Novitates 873, 1-27